Apr 24, 2024
0 comment
Lipogems Completes Enrollment of Final Patient in U.S. FDA IDE ARISE Study Targeting Knee Osteoarthritis Treatment
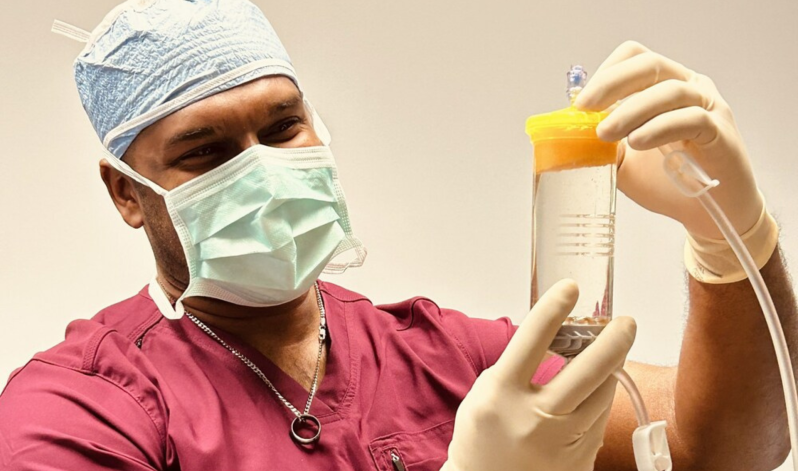
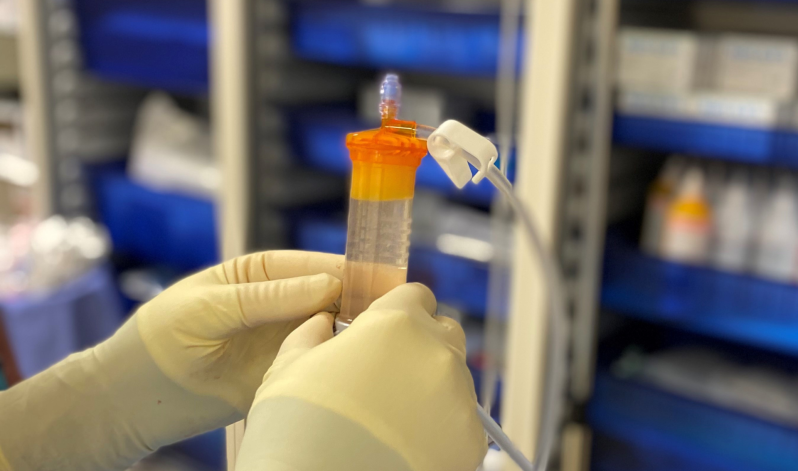
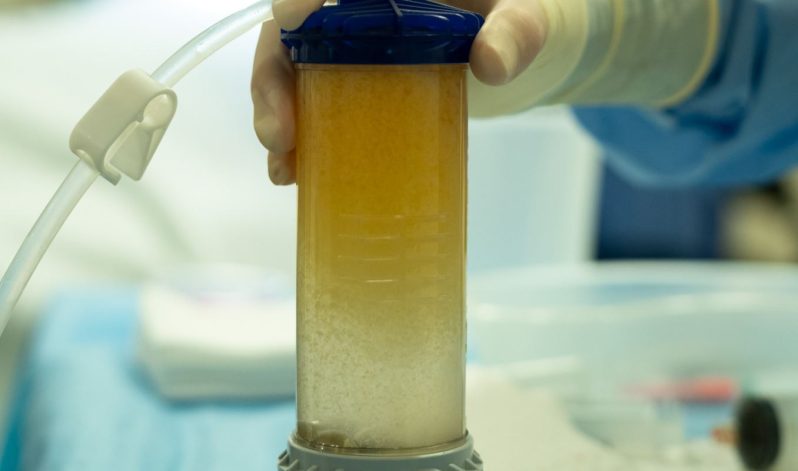
Lipogems, a prominent medical device company renowned for its adipose tissue solutions, has been granted another Investigational Device Exemption (IDE) study by the U.S. Food and Drug Administration (FDA). This second study focuses on the use of MicroFat for the treatment of knee osteoarthritis (OA). We are proud to be the exclusive distributor of Lipogems kits in the UK and able to provide training for orthopaedic purposes.“It is a tremendous accomplishment to complete enrollment in the Lipogems ARISE 1 FDA IDE Study ahead of expectations! I’m proud of our team, the principal investigators and their dedicated teams, as well as our collaboration with Alira Health to achieve this significant milestone in only 14 months,” said Giorgio Ninzoli, Chief Executive Officer, Lipogems International. “Lipogems could be the answer to help the growing global demand of patients suffering from Knee OA by treating the disease in the earlier stages.”, said Carl Llewellyn, Chief Executive Officer, Lipogems USA. “We are confident this study will prove that Lipogems is a safe, effective, natural and minimally invasive option to treat this debilitating disease or delay the need for a knee replacement.”This double-blinded randomised controlled trial commenced in January 2023 at 20 sites across the United States, including respected orthopaedic institutions, enrolling a total of 173 subjects. Following lipoaspiration, patients were administered either MicroFat or corticosteroid injections. The primary endpoints assess improvements in pain and function one year post-injection.For the past decade, Lipogems has held FDA clearance for applications in orthopaedics, arthroscopic surgery, and nine other specialties. The ARISE I and II studies will enable Lipogems to pursue a distinct and specific indication in knee osteoarthritis. Despite being supported by over 160 peer-reviewed publications, the ARISE studies represent Lipogems’ largest endeavors to date.Lipogems International, a privately held medical device company, leverages adipose tissue solutions to sustain or restore patient lifestyles, enhancing quality of life and recovery times. Lipogems’ products find utility across various specialties, notably in orthopaedics, with exploration ongoing into applications and indications in other relevant fields. Lipogems is accessible in 29 countries, embodying the vision of reconnecting patients with their lives through global outreach and scientific excellence.In the UK, the Living Room Health Regenerative Treatments serves as the exclusive distributor of Lipogems kits, bringing cutting-edge medical solutions to orthopaedics, aesthetics, and veterinary specialties. Lipogems, with FDA and CE mark approval, continues to revolutionise treatments across diverse medical domains.For more information about Lipogems and its innovative adipose tissue solutions, visit UnderstandingLipogems.com, or contact us for exclusive access to Lipogems kits in the UK.Read the full article here.